The manufacturing of sulphuric acid is one of the most important chemical inductries at the present time. Sulphuric acid, H2SO4 is a non-volatile diprotic acid. Concentrated sulphuric acid is a viscous colourless liquid. Sulphuric acid is an important chemical used to make other manufactures substances. The uses of sulphuric acid are to manufacture fertilizers, detergents, pesticides, synthetic fibre, paint pigments, as an electrolyte in lead acid accumulators, to remove metal oxides from metal surfaces before electroplating and etc. Sulphuric acid is manufactured by the Contact process in industry. The raw materials used in the Contact process are sulphur or sulphide mineral), air and water. The Contact process involves three steps:
sulphur -> sulphur dioxide -> sulphur trioxide -> sulphuric acid
Step I: Production of sulphur dioxide gas, SO2.
(a) Burning of sulphur in air.
S + O2 --> SO2
(b) Burning of metal sulphide such as zinc sulphide or iron(III) sulphide in air.
2ZnS + 3O2 --> 2SO2 + 2ZnO
Step II: Conversion of sulphur dioxide to sulohur trioxide, SO3
(a) Pure and dry sulphur dioxide with excess dry oxygen(from air) are passed
through a converter.
(b) A high percentage of sulphur dioxide is converted into sulphur trioxide under the following conditions:
(i) The presence of vanadium(V) oxide,V2O5 as a catalyst.
(ii) A temperature of 450’C – 550’C.
(iii) A pressure of one atmosphere.
2SO2 + O2 <--> 2SO3
Step III: Production of sulphuric acid
(a) The sulphur trioxide produced is dissolved in concentrated sulphuric acid to produce oleum, H2S2O7, a viscous liquid.
SO3 + H2SO4 --> H2S2O7
(b) Oleum is then diluted with an equal volume of water to produce concentrated sulphuric acid(98%).
H2S2O7 + H2O --> 2H2SO4

Sulphur trioxide is not dissolved directly in water to produce sulphuric acid.
SO3 + H2O --> H2SO4
This is because:
(a) the solubility of sulphur trioxide in water is low.
(b) The process releases a large amount of heat which will vapourise sulphuric acid to form fumes.
The sulphur dioxide gas is dried and purified before the production of sulphur trioxide gas.This is to remove:
(a) water vapour ( the reaction of water with sulphur trioxide will produce heat that will vapourise the acid)
(b) Contaminants such as arsenic compounds( found in the sulphur or sulphide mineral) which will poison the catalyst and make it ineffective.
The conversion of sulphur dioxide into sulphur trioxide is a reversible process. This means that not all the sulphur dioxide can be concerted into sulphur trioxide. The optimum temperature of 450’C – 550’C, the pressure of 1 atm and the use of V2O5 as catalyst are needed to ensure that a high percentage of sulphur trioxide is formed. Under these conditions, the conversion efficiency is about 98%.
 suspension of colloidal rubber particles. Each rubber particle is made up of rubber polymers covered by a layer of protein membrane. Negative charg
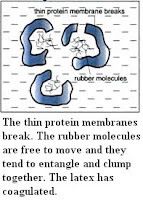
suspension of colloidal rubber particles. Each rubber particle is made up of rubber polymers covered by a layer of protein membrane. Negative charg es are found on the surface of the membrane, making each rubber particle negative charged. The negative-charged rubber particles repel each other, preventing themselves from combining and coagulating.
es are found on the surface of the membrane, making each rubber particle negative charged. The negative-charged rubber particles repel each other, preventing themselves from combining and coagulating.