
A polymer is a large, long-chain molecule formed by joining together thousands of small m on o m er molecules. Polymer can be classified into two groups which are natural polymers and synthetic polymers. Nat ural rubber is one of the example of natural polymers. The monomer unit of natural rubber is isoprene. Its IUPAC name is 2-methylbuta-1,3-diene.
An addition polymerization joins thousands of isoprene unit together to form poly(isoprene) or natural rubber.

The milky fluid obtained from tapped rubber trees is called latex. It consists of an aqueous suspension of colloidal rubber particles. Each rubber particle is made up of rubber polymers covered by a layer of protein membrane. Negative charg
suspension of colloidal rubber particles. Each rubber particle is made up of rubber polymers covered by a layer of protein membrane. Negative charg es are found on the surface of the membrane, making each rubber particle negative charged. The negative-charged rubber particles repel each other, preventing themselves from combining and coagulating.
es are found on the surface of the membrane, making each rubber particle negative charged. The negative-charged rubber particles repel each other, preventing themselves from combining and coagulating.
Acids such as methanoic acid(formic acid) are added to make the latex coagulate. Hydrogen ions from the acid neutralize the negative charges on the surface of the membrane. A neutral rubber particle is formed. When these neutral particles collide with each other, their outer membrane layers break up. The rubber polymers are set free.


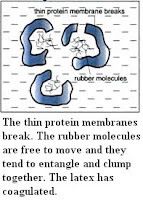
The rubber polymers start to coagulate by combining together to form large lumps of rubber polymers which then precipitate out of the latex solution. Latex can still coagulate if acids are not added. Normally, the latex will coagulate if left overnight. Bacteria from the air slowly attack the protein on the membrane to produce lactic acid. Ionisation of the lactic acid produces hydrogen ions. The hydrogen ions neutralize the negative charges to form neutral rubber particles, allowing coagulation to occur. Alkalis such as solution are added to latex to prevent coagulation. The hydroxide ions from alkali neutralize hydrogen ions produced by lactic acid as a result of bacterial attack on protein. Because there are no hydrogen ions to neutralize the negative charges on the rubber particles, they remain negatively charged and hence cannot combine and coagulate.
Vulcanisation is a manufacturing process discovered by Charles Goodyear in 1839 to convert raw rubber into a tough useful product. In this year, about 1-3% by weight of sulphur is added to raw rubber and the mixture is carefully heated. Sulphur atoms form cross-links between adjacent chains of rubber polymers at the carbon-carbon double bonds. The number of sulphur atoms in the cross-links us usually one to four.
The cross-linking improve the properties of raw rubber, making vulcanized rubber 
(a) a tougher material that is more resistant to oxidation.
(b) more elastic as the cross-linked chains can revert back to their original positions.
(c) more heat resistant which means the vulcanized rubber is less soft and sticky on warming.
(d) less soluble in organic solvent.
 suspension of colloidal rubber particles. Each rubber particle is made up of rubber polymers covered by a layer of protein membrane. Negative charg
suspension of colloidal rubber particles. Each rubber particle is made up of rubber polymers covered by a layer of protein membrane. Negative charg es are found on the surface of the membrane, making each rubber particle negative charged. The negative-charged rubber particles repel each other, preventing themselves from combining and coagulating.
es are found on the surface of the membrane, making each rubber particle negative charged. The negative-charged rubber particles repel each other, preventing themselves from combining and coagulating.




