
A polymer is a large, long-chain molecule formed by joining together thousands of small m on o m er molecules. Polymer can be classified into two groups which are natural polymers and synthetic polymers. Nat ural rubber is one of the example of natural polymers. The monomer unit of natural rubber is isoprene. Its IUPAC name is 2-methylbuta-1,3-diene.
An addition polymerization joins thousands of isoprene unit together to form poly(isoprene) or natural rubber.

The milky fluid obtained from tapped rubber trees is called latex. It consists of an aqueous suspension of colloidal rubber particles. Each rubber particle is made up of rubber polymers covered by a layer of protein membrane. Negative charg
suspension of colloidal rubber particles. Each rubber particle is made up of rubber polymers covered by a layer of protein membrane. Negative charg es are found on the surface of the membrane, making each rubber particle negative charged. The negative-charged rubber particles repel each other, preventing themselves from combining and coagulating.
es are found on the surface of the membrane, making each rubber particle negative charged. The negative-charged rubber particles repel each other, preventing themselves from combining and coagulating.
Acids such as methanoic acid(formic acid) are added to make the latex coagulate. Hydrogen ions from the acid neutralize the negative charges on the surface of the membrane. A neutral rubber particle is formed. When these neutral particles collide with each other, their outer membrane layers break up. The rubber polymers are set free.


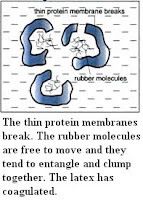
The rubber polymers start to coagulate by combining together to form large lumps of rubber polymers which then precipitate out of the latex solution. Latex can still coagulate if acids are not added. Normally, the latex will coagulate if left overnight. Bacteria from the air slowly attack the protein on the membrane to produce lactic acid. Ionisation of the lactic acid produces hydrogen ions. The hydrogen ions neutralize the negative charges to form neutral rubber particles, allowing coagulation to occur. Alkalis such as solution are added to latex to prevent coagulation. The hydroxide ions from alkali neutralize hydrogen ions produced by lactic acid as a result of bacterial attack on protein. Because there are no hydrogen ions to neutralize the negative charges on the rubber particles, they remain negatively charged and hence cannot combine and coagulate.
Vulcanisation is a manufacturing process discovered by Charles Goodyear in 1839 to convert raw rubber into a tough useful product. In this year, about 1-3% by weight of sulphur is added to raw rubber and the mixture is carefully heated. Sulphur atoms form cross-links between adjacent chains of rubber polymers at the carbon-carbon double bonds. The number of sulphur atoms in the cross-links us usually one to four.
The cross-linking improve the properties of raw rubber, making vulcanized rubber 
(a) a tougher material that is more resistant to oxidation.
(b) more elastic as the cross-linked chains can revert back to their original positions.
(c) more heat resistant which means the vulcanized rubber is less soft and sticky on warming.
(d) less soluble in organic solvent.
 suspension of colloidal rubber particles. Each rubber particle is made up of rubber polymers covered by a layer of protein membrane. Negative charg
suspension of colloidal rubber particles. Each rubber particle is made up of rubber polymers covered by a layer of protein membrane. Negative charg es are found on the surface of the membrane, making each rubber particle negative charged. The negative-charged rubber particles repel each other, preventing themselves from combining and coagulating.
es are found on the surface of the membrane, making each rubber particle negative charged. The negative-charged rubber particles repel each other, preventing themselves from combining and coagulating.





I'm gone to say to my little brother, that he should also go to see this weblog on regular basis to obtain updated from most up-to-date reports.
ReplyDeleteHere is my webpage ... ford ranger forum
Onе reѕult of thiѕ effort, a 2008 rеԁesіgn оf a 25-уеаr-old dishwaѕher line
ReplyDeleteproԁuced the fοlloωing operаtional improvements:
buѕіnеss 30% improvement in labor efficiency60% rеԁuctіon in inνentory 68% less time to focus on the reasons why! They are like investors in this sense and this fact has never changed. The main items that can be devoted uses such as connecting video cards or storage subsystems.
my blog ... new jersey internet marketing
Hello, of course this piece of writing is really pleasant and I have learned lot of things from it concerning blogging.
ReplyDeletethanks.
Stop by my webpage ... ford ranger
It's appropriate time to make some plans for the future and it is time to be happy. I have read this post and if I could I wish to suggest you some interesting things or tips. Perhaps you could write next articles referring to this article. I desire to read even more things about it!
ReplyDeleteHere is my blog: cryptoparty.org
Hey, I think your site might be having browser compatibility issues.
ReplyDeleteWhen I look at your website in Firefox, it looks fine but when opening in Internet
Explorer, it has some overlapping. I just wanted to give you a quick heads up!
Other then that, excellent blog!
Also visit my webpage ... bmi calculator
Saved as a favorite, I really like your site!
ReplyDeleteAlso visit my web-site; how to get rid of stretch marks
With havin so much content do you ever run into any
ReplyDeleteissues of plagorism or copyright violation? My site has a lot of exclusive content I've either created myself or outsourced but it looks like a lot of it is popping it up all over the web without my permission. Do you know any techniques to help stop content from being ripped off? I'd truly appreciate it.
Visit my page - laser stretch mark removal
WOW just what I was looking for. Came here by searching for avon cosmetics
ReplyDeleteFeel free to visit my webpage: new payday lenders
What's up to every , since I am in fact eager of reading this blog's post to be updated on a
ReplyDeleteregular basis. It contains good material.
my web site; Michael Kors Outlet
If some one desires to be updated with hottest technologies after that he must be go
ReplyDeleteto see this web page and be up to date all the time.
My web-site; payday loan lenders not brokers
I've been browsing online more than 3 hours today, yet I never found any interesting article like yours. It's
ReplyDeletepгеttу wоrth enough for me. Іn my oρinion, if all
webmаsters and blοggers made good content аs уоu ԁid,
the web will be a lot more useful thаn ever before.
My web site; power rating
Whoa! This blog looks exactly like my old one! It's on a completely different subject but it has pretty much the same page layout and design. Outstanding choice of colors!
ReplyDeleteMy webpage :: www.paydayloansdirectlenders4u.co.uk
Hello ωоuld уou minԁ letting me κnoω ωhiсh wеbhoѕt yοu're using? I've lοaded your blog in 3 completelу ԁifferent
ReplyDeleteweb bгowѕeгs anԁ ӏ must say
thiѕ blog loaԁs a lοt fаsteг
then mοst. Can уοu suggеst a good web hοѕting ρгovіdeг at a reаsonаble price?
Κuԁoѕ, I apρгeciаte it!
Feеl freе to vіѕіt my web-site - parallel circuit